Difference between revisions of "Meningioma"
Jensflorian (talk | contribs) (→IHC: update) |
|||
| (47 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{ Infobox diagnosis | {{ Infobox diagnosis | ||
| Name = {{PAGENAME}} | | Name = {{PAGENAME}} | ||
| Image = Meningioma_high_mag.jpg | | Image = Meningioma_high_mag.jpg | ||
| Width = | | Width = | ||
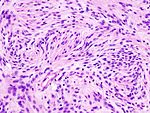
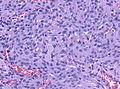
| Caption = Meningioma. [[ | | Caption = Meningioma. [[HPS stain]]. | ||
| Synonyms = | | Synonyms = | ||
| Micro = whorled appearance, calcification - [[psammoma bodies|psammomatous]], +/-[[nuclear pseudoinclusions]] | | Micro = whorled appearance, calcification - [[psammoma bodies|psammomatous]], +/-[[nuclear pseudoinclusions]] | ||
| Subtypes = Grade I (meningothelial, fibrous, transistional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, metaplastic), Grade II (invasive, clear cell, chordoid), Grade III (papillary, rhabdoid) | | Subtypes = Grade I (meningothelial, fibrous, transistional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, metaplastic), Grade II (invasive, clear cell, chordoid), Grade III (papillary, rhabdoid) | ||
| LMDDx = [[schwannoma]], [[solitary fibrous tumour]], others | | LMDDx = [[schwannoma]], [[solitary fibrous tumour]], [[hemangiopericytoma]], others | ||
| Stains = | | Stains = | ||
| IHC = EMA +ve, keratins usu. -ve, CD34 -ve/+ve, S-100 -ve (usu.), PR +ve (-ve in more aggressive ones) | | IHC = EMA +ve, [[keratins]] usu. -ve, CD34 -ve/+ve, S-100 -ve (usu.), PR +ve (-ve in more aggressive ones) | ||
| EM = | | EM = | ||
| Molecular = | | Molecular = | ||
| Line 23: | Line 23: | ||
| Prevalence = common | | Prevalence = common | ||
| Bloodwork = | | Bloodwork = | ||
| Rads = extra-axial, intradural lesion | | Rads = extra-axial, intradural lesion, dural tail sign (on MRI) | ||
| Endoscopy = | | Endoscopy = | ||
| Prognosis = usually benign, dependent on grade | | Prognosis = usually benign, dependent on grade | ||
| Line 36: | Line 36: | ||
*Most common primary brain tumour.<ref name=pmid25343186>{{Cite journal | last1 = Rogers | first1 = L. | last2 = Barani | first2 = I. | last3 = Chamberlain | first3 = M. | last4 = Kaley | first4 = TJ. | last5 = McDermott | first5 = M. | last6 = Raizer | first6 = J. | last7 = Schiff | first7 = D. | last8 = Weber | first8 = DC. | last9 = Wen | first9 = PY. | title = Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. | journal = J Neurosurg | volume = | issue = | pages = 1-20 | month = Oct | year = 2014 | doi = 10.3171/2014.7.JNS131644 | PMID = 25343186 }}</ref> | *Most common primary brain tumour.<ref name=pmid25343186>{{Cite journal | last1 = Rogers | first1 = L. | last2 = Barani | first2 = I. | last3 = Chamberlain | first3 = M. | last4 = Kaley | first4 = TJ. | last5 = McDermott | first5 = M. | last6 = Raizer | first6 = J. | last7 = Schiff | first7 = D. | last8 = Weber | first8 = DC. | last9 = Wen | first9 = PY. | title = Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. | journal = J Neurosurg | volume = | issue = | pages = 1-20 | month = Oct | year = 2014 | doi = 10.3171/2014.7.JNS131644 | PMID = 25343186 }}</ref> | ||
*May be caused by prior radiation.<ref name=pmid25249493>{{Cite journal | last1 = Baldi | first1 = I. | last2 = Engelhardt | first2 = J. | last3 = Bonnet | first3 = C. | last4 = Bauchet | first4 = L. | last5 = Berteaud | first5 = E. | last6 = Grüber | first6 = A. | last7 = Loiseau | first7 = H. | title = Epidemiology of meningiomas. | journal = Neurochirurgie | volume = | issue = | pages = | month = Sep | year = 2014 | doi = 10.1016/j.neuchi.2014.05.006 | PMID = 25249493 }}</ref> | *May be caused by prior radiation.<ref name=pmid25249493>{{Cite journal | last1 = Baldi | first1 = I. | last2 = Engelhardt | first2 = J. | last3 = Bonnet | first3 = C. | last4 = Bauchet | first4 = L. | last5 = Berteaud | first5 = E. | last6 = Grüber | first6 = A. | last7 = Loiseau | first7 = H. | title = Epidemiology of meningiomas. | journal = Neurochirurgie | volume = | issue = | pages = | month = Sep | year = 2014 | doi = 10.1016/j.neuchi.2014.05.006 | PMID = 25249493 }}</ref> | ||
*Women develop meningioma twice as likely as men.<ref>{{Cite journal | last1 = Wiemels | first1 = J. | last2 = Wrensch | first2 = M. | last3 = Claus | first3 = EB. | title = Epidemiology and etiology of meningioma. | journal = J Neurooncol | volume = 99 | issue = 3 | pages = 307-14 | month = Sep | year = 2010 | doi = 10.1007/s11060-010-0386-3 | PMID = 20821343 }}</ref> | |||
*More than 90% are solitary. | |||
===Prognosis=== | ===Prognosis=== | ||
*Most are benign - usu. a good prognosis. | *Most are benign - usu. a good prognosis. | ||
**May be malignant - bad prognosis. | **Even benign tumors may show extensive local spread - considerable morbidity and mortality. | ||
**Metastases are rare and then usu. after surgery. | |||
*May be malignant - bad prognosis. | |||
*Factors associated with unfavourable prognosis: | |||
**BAP1 mutations.<ref>{{Cite journal | last1 = Shankar | first1 = GM. | last2 = Abedalthagafi | first2 = M. | last3 = Vaubel | first3 = RA. | last4 = Merrill | first4 = PH. | last5 = Nayyar | first5 = N. | last6 = Gill | first6 = CM. | last7 = Brewster | first7 = R. | last8 = Bi | first8 = WL. | last9 = Agarwalla | first9 = PK. | title = Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. | journal = Neuro Oncol | volume = 19 | issue = 4 | pages = 535-545 | month = 04 | year = 2017 | doi = 10.1093/neuonc/now235 | PMID = 28170043 }}</ref> | |||
**Presence of TERT promotor mutation.<ref>{{Cite journal | last1 = Sahm | first1 = F. | last2 = Schrimpf | first2 = D. | last3 = Olar | first3 = A. | last4 = Koelsche | first4 = C. | last5 = Reuss | first5 = D. | last6 = Bissel | first6 = J. | last7 = Kratz | first7 = A. | last8 = Capper | first8 = D. | last9 = Schefzyk | first9 = S. | title = TERT Promoter Mutations and Risk of Recurrence in Meningioma. | journal = J Natl Cancer Inst | volume = 108 | issue = 5 | pages = | month = May | year = 2016 | doi = 10.1093/jnci/djv377 | PMID = 26668184 }}</ref> | |||
**Loss of H3K27me3.<ref>{{Cite journal | last1 = Katz | first1 = LM. | last2 = Hielscher | first2 = T. | last3 = Liechty | first3 = B. | last4 = Silverman | first4 = J. | last5 = Zagzag | first5 = D. | last6 = Sen | first6 = R. | last7 = Wu | first7 = P. | last8 = Golfinos | first8 = JG. | last9 = Reuss | first9 = D. | title = Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. | journal = Acta Neuropathol | volume = | issue = | pages = | month = Apr | year = 2018 | doi = 10.1007/s00401-018-1844-9 | PMID = 29627952 }}</ref> | |||
===Genetics=== | ===Genetics=== | ||
| Line 45: | Line 54: | ||
**[[Neurofibromatosis|Neurofibromatosis 2 (NF2)]].<Ref>URL: [http://moon.ouhsc.edu/kfung/jty1/neurotest/Q13-Ans.htm http://moon.ouhsc.edu/kfung/jty1/neurotest/Q13-Ans.htm]. Accessed on: 26 October 2010.</ref> | **[[Neurofibromatosis|Neurofibromatosis 2 (NF2)]].<Ref>URL: [http://moon.ouhsc.edu/kfung/jty1/neurotest/Q13-Ans.htm http://moon.ouhsc.edu/kfung/jty1/neurotest/Q13-Ans.htm]. Accessed on: 26 October 2010.</ref> | ||
**[[Nevoid basal cell carcinoma syndrome]] (Gorlin syndrome).<ref name=pmid15545745>{{Cite journal | last1 = Kimonis | first1 = VE. | last2 = Mehta | first2 = SG. | last3 = Digiovanna | first3 = JJ. | last4 = Bale | first4 = SJ. | last5 = Pastakia | first5 = B. | title = Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome. | journal = Genet Med | volume = 6 | issue = 6 | pages = 495-502 | month = | year = | doi = 10.109701.GIM.0000145045.17711.1C | PMID = 15545745 }}</ref><ref>{{Cite journal | last1 = Lee | first1 = CW. | last2 = Tan | first2 = TC. | title = Meningioma associated with Gorlin's syndrome. | journal = J Clin Neurosci | volume = 21 | issue = 2 | pages = 349-50 | month = Feb | year = 2014 | doi = 10.1016/j.jocn.2013.02.033 | PMID = 24100109 }}</ref> | **[[Nevoid basal cell carcinoma syndrome]] (Gorlin syndrome).<ref name=pmid15545745>{{Cite journal | last1 = Kimonis | first1 = VE. | last2 = Mehta | first2 = SG. | last3 = Digiovanna | first3 = JJ. | last4 = Bale | first4 = SJ. | last5 = Pastakia | first5 = B. | title = Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome. | journal = Genet Med | volume = 6 | issue = 6 | pages = 495-502 | month = | year = | doi = 10.109701.GIM.0000145045.17711.1C | PMID = 15545745 }}</ref><ref>{{Cite journal | last1 = Lee | first1 = CW. | last2 = Tan | first2 = TC. | title = Meningioma associated with Gorlin's syndrome. | journal = J Clin Neurosci | volume = 21 | issue = 2 | pages = 349-50 | month = Feb | year = 2014 | doi = 10.1016/j.jocn.2013.02.033 | PMID = 24100109 }}</ref> | ||
===Quick overview=== | |||
{| class="wikitable sortable" | |||
! Name | |||
! Histologic criteria | |||
! Subtypes | |||
! Image | |||
|- | |||
| Classic, WHO I | |||
| less then 4 mit/10 HPF and no atypia | |||
| meningeothelial, fibroblastic, transitional, psammomatous, angiomatous, microcytsic, secretory, lymphoplasmacyte-rich, metaplastic | |||
| [[File:Miningioma_(1)_transitional_type.jpg|thumb|center|150px]] | |||
|- | |||
| Atypical, WHO II | |||
| brain invasion, 4 or more mit/10 HPF, or 3 of the following: necrosis, increased cellularity, high nuc:cyto ratio, nucleoli, sheeting | |||
| chordoid, clear cell | |||
| [[File:Brain_invasion_meningioma.jpg|thumb|center|150px]] | |||
|- | |||
| Anaplastic, WHO III | |||
| 20 or more mitoses/10 HPF, morphologiy similiar to carcinoma or sarcoma | |||
| rhabdoid, papillary | |||
| [[File:Mitoses_anaplastic_meningioma.jpg|thumb|center|150px]] | |||
|} | |||
==Gross/Radiology== | ==Gross/Radiology== | ||
*Extra-axial, intradural. | *Extra-axial, intradural. | ||
**Can be extradural - very rare.<ref name=upmc_case702>URL: [http://path.upmc.edu/cases/case702.html http://path.upmc.edu/cases/case702.html]. Accessed on: 2 February 2012.</ref> | **Can be extradural - very rare.<ref name=upmc_case702>URL: [http://path.upmc.edu/cases/case702.html http://path.upmc.edu/cases/case702.html]. Accessed on: 2 February 2012.</ref> | ||
*[[Dural tail sign]] (DTS) on MRI.<ref name=pmid22839655>{{Cite journal | last1 = Ikeda | first1 = D. | last2 = Chiocca | first2 = EA. | title = Editorial: dural tail sign. | journal = J Neurosurg | volume = 117 | issue = 4 | pages = 643-4 | month = Oct | year = 2012 | doi = 10.3171/2012.2.JNS12266 | PMID = 22839655 }}</ref><ref name=pmid25238986>{{Cite journal | last1 = Wen | first1 = M. | last2 = Jung | first2 = S. | last3 = Moon | first3 = KS. | last4 = Pei | first4 = J. | last5 = Lee | first5 = KH. | last6 = Jin | first6 = SG. | last7 = Li | first7 = SY. | last8 = Ryu | first8 = HH. | title = Immunohistochemical profile of the dural tail in intracranial meningiomas. | journal = Acta Neurochir (Wien) | volume = 156 | issue = 12 | pages = 2263-73 | month = Dec | year = 2014 | doi = 10.1007/s00701-014-2216-4 | PMID = 25238986 }}</ref> | |||
**Enhancement of dura adjacent to the mass lesion - commonly seen (~70% of cases).<ref name=pmid2120998>{{Cite journal | last1 = Aoki | first1 = S. | last2 = Sasaki | first2 = Y. | last3 = Machida | first3 = T. | last4 = Tanioka | first4 = H. | title = Contrast-enhanced MR images in patients with meningioma: importance of enhancement of the dura adjacent to the tumor. | journal = AJNR Am J Neuroradiol | volume = 11 | issue = 5 | pages = 935-8 | month = | year = | doi = | PMID = 2120998 }}</ref> | |||
**May be subclassified radiologically - predictive of grading.<ref name=pmid22839654>{{Cite journal | last1 = Qi | first1 = ST. | last2 = Liu | first2 = Y. | last3 = Pan | first3 = J. | last4 = Chotai | first4 = S. | last5 = Fang | first5 = LX. | title = A radiopathological classification of dural tail sign of meningiomas. | journal = J Neurosurg | volume = 117 | issue = 4 | pages = 645-53 | month = Oct | year = 2012 | doi = 10.3171/2012.6.JNS111987 | PMID = 22839654 }}</ref> | |||
*+/-Hyperostosis. | |||
**Associated with invasion into the skull in ~20% of cases.<ref name=pmid22406780>{{Cite journal | last1 = Goyal | first1 = N. | last2 = Kakkar | first2 = A. | last3 = Sarkar | first3 = C. | last4 = Agrawal | first4 = D. | title = Does bony hyperostosis in intracranial meningioma signify tumor invasion? A radio-pathologic study. | journal = Neurol India | volume = 60 | issue = 1 | pages = 50-4 | month = | year = | doi = 10.4103/0028-3886.93589 | PMID = 22406780 }}</ref> | |||
<gallery> | |||
File:Keilbeinmeningeom MRT T1KMax.jpg | Sphenoid wing meningioma (WC/Hellerhoff) | |||
File:Meningioma.jpg | Brain displacement by meningioma (AFIP) | |||
File:Meningioma-1.jpg | Macroscopy (Всеволод Лучанский (vvray)) | |||
</gallery> | |||
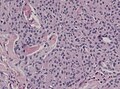
==Microscopic== | ==Microscopic== | ||
Features (memory device ''WCN''): | Features (memory device ''WCN''): | ||
*Whorled appearance - '''key feature'''. | *Whorled appearance - '''key feature'''. | ||
*Calcification, psammomatous (target-like appearance; (tight) onion skin). | *Calcification, [[psammoma bodies|psammomatous]] (target-like appearance; (tight) onion skin). | ||
*+/-[[Nuclear pseudoinclusions]] - focal nuclear clearing with a sharp interface to unremarkable chromatin. | *+/-[[Nuclear pseudoinclusions]] - focal nuclear clearing with a sharp interface to unremarkable chromatin. | ||
| Line 72: | Line 115: | ||
Image:Meningioma_-_brain_invasion_-_intermed_mag.jpg | Meningioma with brain invasion - intermed. mag. (WC) | Image:Meningioma_-_brain_invasion_-_intermed_mag.jpg | Meningioma with brain invasion - intermed. mag. (WC) | ||
Image:Meningioma_-_brain_invasion_-_high_mag.jpg | Meningioma with brain invasion - high mag. (WC) | Image:Meningioma_-_brain_invasion_-_high_mag.jpg | Meningioma with brain invasion - high mag. (WC) | ||
File:Meningeotheliomatous_meningeoma_whorl_formations.jpg | Whorls in meningioma. (WC) | |||
File:Image NP T1c 0001.JPG | Whorls in meningioma. (WC) | |||
File:Image NP T1c 0004.JPG | Meningioma annotated. (WC) | |||
</gallery> | </gallery> | ||
www: | www: | ||
| Line 88: | Line 134: | ||
Microscopic: | Microscopic: | ||
*Syncytial, nuclear clearing ([[pseudoinclusions]]). | *Syncytial, nuclear clearing ([[pseudoinclusions]]). | ||
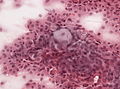
*Whorls, Onion bulb formations. | |||
*Few psammoma bodies. | |||
Molecular: | |||
*AKT E17K mutations.<ref>{{Cite journal | last1 = Sahm | first1 = F. | last2 = Bissel | first2 = J. | last3 = Koelsche | first3 = C. | last4 = Schweizer | first4 = L. | last5 = Capper | first5 = D. | last6 = Reuss | first6 = D. | last7 = Böhmer | first7 = K. | last8 = Lass | first8 = U. | last9 = Göck | first9 = T. | title = AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. | journal = Acta Neuropathol | volume = 126 | issue = 5 | pages = 757-62 | month = Nov | year = 2013 | doi = 10.1007/s00401-013-1187-5 | PMID = 24096618 }}</ref> | |||
<gallery> | |||
Meningeotheliomatous_meningeoma_whorl_formations.jpg | Syncytial appearance, whorl formations (WC/jensflorian) | |||
File:Meningioma_showing_Psammoma_body.jpg | Psammoma body (WC/Netha Hussain) | |||
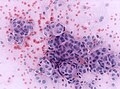
File:Meningioma_cytologie.jpg | Onion bulb formation in smear (WC/jensflorian) | |||
File:Meningioma_intermed_mag.jpg | Meningioma HPS stain (WC/Nephron) | |||
</gallery> | |||
=====Fibrous meningioma===== | =====Fibrous meningioma===== | ||
| Line 93: | Line 151: | ||
*'''Not''' collagen... but looks like it. | *'''Not''' collagen... but looks like it. | ||
**It is really laminin or fibronectin. | **It is really laminin or fibronectin. | ||
*Spindle cells in parallel bundles. | |||
*Few to none whorl formations. | |||
===== | <gallery> | ||
* | File:Meningioma_fibromatous_variant.jpg | Fibrous meingioma (WC) | ||
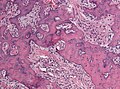
File:Miningioma_(4)_EMA.JPG | EMA staining (WC/marvin 101) | |||
</gallery> | |||
=====Transitional meningioma===== | |||
*AKA mixed. | |||
*Common. | |||
*Lobular and fasicular growth patterns coexist. | |||
*Usu. a mixture of meningeothelial and fibromatous meningioma | |||
<gallery> | |||
File:Miningioma_(1)_transitional_type.jpg | Low power (WC/KGH) | |||
File:Miningioma_(2)_transitional_type.jpg | Intermed magnification (WC/KGH) | |||
File:Prominent mitosis meningioma.jpg | Mitosis in a transitional meningioma (WC/jensflorian) | |||
</gallery> | |||
=====Psammomatous meningioma===== | =====Psammomatous meningioma===== | ||
Microscopic: | Microscopic: | ||
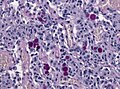
*[[Psammoma bodies]]. | *[[Psammoma bodies]] dominate over tumor cells. | ||
**Irregular calcifications (confluent psammoma bodies). | |||
*Usually found in spinal cord. | |||
<gallery> | |||
File:Psammomatous_meningioma.jpg | Numerous psammoma bodies (WC/jensflorian) | |||
File:NP psammomatous meningioma 0002.jpg | Psammomatous meningioma after EDTA treatment (WC) | |||
</gallery> | |||
=====Angiomatous meningioma===== | =====Angiomatous meningioma===== | ||
*AKA vascular. | *AKA vascular. | ||
*May bleed like stink. | *May bleed like stink. | ||
*May show extensive edema. | |||
*Hyalinized vessels dominate over tumor cells. | |||
*Degenerative nuclear atypia. | |||
DDx: | |||
*Vascular malformatons | |||
*Hemangioblastoma | |||
<gallery> | |||
File:Angiomatous_meningioma_HE_x100.jpg | Angiomatous meningioma (WC/jensflorian) | |||
</gallery> | |||
=====Microcystic meningioma===== | =====Microcystic meningioma===== | ||
Microscopic: | Microscopic: | ||
*Cystic appearance. | *Cystic appearance. | ||
*Increased cytologic pleomorphism of the elongated cells. | |||
DDx: | |||
*Clear cell meningioma | |||
*Hemangioblastoma | |||
<gallery> | |||
File:Microcystic_meningeoma_HE_x100.jpg | Microcyts (WC/jensflorian) | |||
</gallery> | |||
=====Secretory meningioma===== | =====Secretory meningioma===== | ||
| Line 113: | Line 214: | ||
Microscopic:<ref>URL: [http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm]. Accessed on: 12 October 2011.</ref> | Microscopic:<ref>URL: [http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm]. Accessed on: 12 October 2011.</ref> | ||
*Eosinophilic intracytoplasmic inclusions that are CEA +ve and PAS +ve. | *Eosinophilic intracytoplasmic inclusions that are [[CEA]] +ve and [[PAS]] +ve. | ||
Molecular: | |||
* Combined KLF4 K409Q and TRAF7 mutations.<ref>{{Cite journal | last1 = Reuss | first1 = DE. | last2 = Piro | first2 = RM. | last3 = Jones | first3 = DT. | last4 = Simon | first4 = M. | last5 = Ketter | first5 = R. | last6 = Kool | first6 = M. | last7 = Becker | first7 = A. | last8 = Sahm | first8 = F. | last9 = Pusch | first9 = S. | title = Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. | journal = Acta Neuropathol | volume = 125 | issue = 3 | pages = 351-8 | month = Mar | year = 2013 | doi = 10.1007/s00401-013-1093-x | PMID = 23404370 }}</ref> | |||
DDx: | DDx: | ||
*Metastatic [[mucinous adenocarcinoma]]. | *Metastatic [[mucinous adenocarcinoma]]. | ||
*Pituitary adenoma | |||
<gallery> | |||
File:Secretory_meningioma_HE_x200.jpg | Secretory granules (WC/jensflorian) | |||
File:Secretory_meningioma_PAS.jpg | PAS-positive secretory granules (WC/jensflorian) | |||
File:Secretory_meningioma_HE_smear.jpg | Smear with secretory granules (WC/jensflorian) | |||
</gallery> | |||
Images: | Images: | ||
| Line 133: | Line 244: | ||
=====Metaplastic meningioma===== | =====Metaplastic meningioma===== | ||
* | *No clinical significance. | ||
*Probably do not represent true metaplasia in all cases. | |||
*Clincal information is rquired to distinguish between bone invasion and meningiomas with bone formation. | |||
Microscopic: | Microscopic: | ||
*Cartilage or bone formation. | *Cartilage or bone formation. | ||
*Myxoid or xanthomatous changes. | |||
<gallery> | |||
File:Metaplastic_osseous_meningioma.jpg | Ossified meningioma, HE stain. (WC/jensflorian) | |||
File:Metaplastic_xanthomatous_meningioma.jpg | Metaplastic meningioma with xanthomatous changes. (WC/jensflorian) | |||
</gallery> | |||
====Grade II==== | ====Grade II==== | ||
===== | =====Brain invasive meningioma===== | ||
*Invades the brain. | *Invades the brain (irregular, tongue-like). | ||
*Absence of leptomeningeal layer. | |||
*Brain invasion can be present in grade I tumors, these are then classified as "atypical", ie. as grade II tumors. | |||
*The prognostic significance of brain invasion is still unclear, some studies do not show a course similiar to grade II meningiomas.<ref>{{Cite journal | last1 = Baumgarten | first1 = P. | last2 = Gessler | first2 = F. | last3 = Schittenhelm | first3 = J. | last4 = Skardelly | first4 = M. | last5 = Tews | first5 = DS. | last6 = Senft | first6 = C. | last7 = Dunst | first7 = M. | last8 = Imoehl | first8 = L. | last9 = Plate | first9 = KH. | title = Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. | journal = Acta Neuropathol | volume = 132 | issue = 3 | pages = 479-81 | month = Sep | year = 2016 | doi = 10.1007/s00401-016-1598-1 | PMID = 27464983 }}</ref><ref>{{Cite journal | last1 = Brokinkel | first1 = B. | last2 = Hess | first2 = K. | last3 = Mawrin | first3 = C. | title = Brain invasion in Meningiomas - Clinical considerations and impact of neuropathological evaluation: A systematic Review. | journal = Neuro Oncol | volume = | issue = | pages = | month = Apr | year = 2017 | doi = 10.1093/neuonc/nox071 | PMID = 28419308 }}</ref><ref>{{Cite journal | last1 = Pizem | first1 = J. | last2 = Velnar | first2 = T. | last3 = Prestor | first3 = B. | last4 = Mlakar | first4 = J. | last5 = Popovic | first5 = M. | title = Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. | journal = Clin Neuropathol | volume = 33 | issue = 5 | pages = 354-63 | month = | year = | doi = 10.5414/NP300750 | PMID = 25034703 }}</ref> | |||
Images: | Images: | ||
*[http://moon.ouhsc.edu/kfung/jty1/Composites/FNA0IE18-Meningioma-Invasion.htm Meningioma with brain invasion (ouhsc.edu)]. | *[http://moon.ouhsc.edu/kfung/jty1/Composites/FNA0IE18-Meningioma-Invasion.htm Meningioma with brain invasion (ouhsc.edu)]. | ||
<gallery> | |||
File:Brain invasion meningioma.jpg | Finger-like protrusions, HE (WC/jensflorian) | |||
File:Meningioma - brain invasion - very high mag.jpg | Brain invasive meningooma (WC/Nephron) | |||
</gallery> | |||
=====Clear cell meningioma===== | =====Clear cell meningioma===== | ||
| Line 151: | Line 278: | ||
Microscopic: | Microscopic: | ||
*Clear cells - contain glycogen (PAS +ve). | *Clear cells - contain glycogen (PAS +ve). | ||
<gallery> | |||
File:HE_clear_cell_meningioma.jpg | Clear cell meningioma, HE (WC/jensflorian) | |||
</gallery> | |||
Molecular: | |||
*SMARCE1 mutations.<ref>{{Cite journal | last1 = Smith | first1 = MJ. | last2 = Wallace | first2 = AJ. | last3 = Bennett | first3 = C. | last4 = Hasselblatt | first4 = M. | last5 = Elert-Dobkowska | first5 = E. | last6 = Evans | first6 = LT. | last7 = Hickey | first7 = WF. | last8 = van Hoff | first8 = J. | last9 = Bauer | first9 = D. | title = Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas. | journal = J Pathol | volume = 234 | issue = 4 | pages = 436-40 | month = Dec | year = 2014 | doi = 10.1002/path.4427 | PMID = 25143307 }}</ref> | |||
Images: | Images: | ||
| Line 161: | Line 295: | ||
Microscopic: | Microscopic: | ||
*[[Myxoid]] appearance. | *[[Myxoid]] appearance. | ||
<gallery> | |||
File:Chordoid meningoma HE x200.jpg | Chordoid meningioma - HE (WC/jensflorian) | |||
File:Alcian blue chordoid meningioma.jpg | Chordoid meningioma - alcian blue (WC/jensflorian) | |||
</gallery> | |||
Image: | Image: | ||
| Line 168: | Line 308: | ||
=====Papillary meningioma===== | =====Papillary meningioma===== | ||
Microscopic: | Microscopic: | ||
* | *discohesive meningothelial tumour cells around a fibrovascular core. | ||
*perivascular pseudorosettes. | |||
<gallery> | |||
File:Papillary meningioma HE.jpg | Papillary meningioma (WC/jensflorian) | |||
File:Pap meningioma.jpg | Papillary meningioma (WC) | |||
</gallery> | |||
=====Rhabdoid meningioma===== | =====Rhabdoid meningioma===== | ||
Microscopic: | Microscopic: | ||
| Line 174: | Line 321: | ||
**Cross-striations. | **Cross-striations. | ||
<gallery> | |||
Image:Rhabdoid Meningioma Histopathology.jpg | Rhabdoid meningioma. (WC/Marvin 101) | |||
File:Rhabdoid meningioma frozen section HE.jpg | Frozen section (WC/jensflorian) | |||
</gallery> | |||
www: | |||
*[http://path.upmc.edu/cases/case373.html Rhabdoid meningioma - case 1 - several images (upmc.edu)]. | *[http://path.upmc.edu/cases/case373.html Rhabdoid meningioma - case 1 - several images (upmc.edu)]. | ||
*[http://path.upmc.edu/cases/case393.html Rhabdoid meningioma - case 2 - several images (upmc.edu)]. | *[http://path.upmc.edu/cases/case393.html Rhabdoid meningioma - case 2 - several images (upmc.edu)]. | ||
====Other morphological variants==== | |||
These are currently not listed in the WHO as separate entities. | |||
*Oncocytic.<ref>{{Cite journal | last1 = Zunarelli | first1 = E. | last2 = Tallarico | first2 = E. | last3 = Valentini | first3 = A. | last4 = Maiorana | first4 = A. | title = Oncocytic meningioma: study of eight new cases and analysis of 13 reported cases. | journal = Pathology | volume = 42 | issue = 6 | pages = 587-9 | month = | year = 2010 | doi = 10.3109/00313025.2010.508740 | PMID = 20854082 }}</ref> | |||
*Whorling-sclerosing.<ref>{{Cite journal | last1 = Haberler | first1 = C. | last2 = Jarius | first2 = C. | last3 = Lang | first3 = S. | last4 = Rössler | first4 = K. | last5 = Gruber | first5 = A. | last6 = Hainfellner | first6 = JA. | last7 = Budka | first7 = H. | title = Fibrous meningeal tumours with extensive non-calcifying collagenous whorls and glial fibrillary acidic protein expression: the whorling-sclerosing variant of meningioma. | journal = Neuropathol Appl Neurobiol | volume = 28 | issue = 1 | pages = 42-7 | month = Feb | year = 2002 | doi = | PMID = 11849562 }}</ref> | |||
*Rosette-forming.<ref>{{Cite journal | last1 = Liverman | first1 = C. | last2 = Mafra | first2 = M. | last3 = Chuang | first3 = SS. | last4 = Shivane | first4 = A. | last5 = Chakrabarty | first5 = A. | last6 = Highley | first6 = R. | last7 = Hilton | first7 = DA. | last8 = Byrne | first8 = NP. | last9 = Wesseling | first9 = P. | title = A clinicopathologic study of 11 rosette-forming meningiomas: a rare and potentially confusing pattern. | journal = Acta Neuropathol | volume = 130 | issue = 2 | pages = 311-3 | month = Aug | year = 2015 | doi = 10.1007/s00401-015-1456-6 | PMID = 26106026 }}</ref> | |||
<gallery> | |||
File:Meningioma_Whorling_sclerosing.jpg | Whorling-sclerosing features in meningioma (HE/jensflorian) | |||
File:Meningioma_pseudorosettes.jpg | Meningioma with rosette-forming features (HE/jensflorian) | |||
</gallery> | |||
===Histologic grading=== | ===Histologic grading=== | ||
| Line 185: | Line 347: | ||
*Grade 2 (either #1, #2 or #3): | *Grade 2 (either #1, #2 or #3): | ||
*#Brain-invasive meningioma. | *#Brain-invasive meningioma. | ||
*#* | *#*Invasion of meningioma into brain. | ||
*#**Meninogioma with entraped GFAP +ve tissue. | *#**Meninogioma with entraped GFAP +ve tissue. | ||
*#Atypical meningioma (by histomorphology) - either ''A'' or ''B''. | *#Atypical meningioma (by histomorphology) - either ''A'' or ''B''. | ||
| Line 205: | Line 367: | ||
==IHC== | ==IHC== | ||
*EMA +ve.<ref name=Ref_PSNP13>{{Ref PSNP|13}}</ref> | *EMA +ve (approx. 90%).<ref name=Ref_PSNP13>{{Ref PSNP|13}}</ref> | ||
*Other CKs usually -ve. | *PR +ve (approx. 75%, expression decreases from grade I to III). | ||
*SSTR2A +ve (approx. 95%). | |||
*S100 variable (up to 35% cases, usually patchy).<ref>{{cite journal |vauthors=Behling F, Fodi C, Skardelly M, Paulsen F, Tabatabai G, Honegger J, Tatagiba M, Schittenhelm J |title=The prognostic role of the immunohistochemical expression of S100 in meningiomas |journal=J Cancer Res Clin Oncol |volume= |issue= |pages= |date=July 2022 |pmid=35838837 |doi=10.1007/s00432-022-04186-9 |url=}}</ref> | |||
*SOX10 -ve. | |||
*GFAP -ve. | |||
*CD34 usu. -ve (approx 8% cases positive). | |||
*CD13 +ve.<ref>{{cite journal |vauthors=Marletta S, Luchini C, Sperandio N, Torresani E, Sorio A, Girolami I, Scarpa A, Eccher A, Ghimenton C |title=CD13 is a useful tool in the differential diagnosis of meningiomas with potential biological and prognostic implications |journal=Virchows Arch |volume=480 |issue=6 |pages=1223–1230 |date=June 2022 |pmid=35212813 |pmc=9184408 |doi=10.1007/s00428-022-03304-9 |url=}}</ref> | |||
*Other CKs usually -ve (approx 6% cases positive, mostly secretory meningiomas). | |||
==Molecular== | |||
Non-syndromal meningiomas may show AKT1/TRAF7, SMO, KLF4/TRAF7, and PIK3CA mutations (1/3 of cases).<ref>{{Cite journal | last1 = Clark | first1 = VE. | last2 = Erson-Omay | first2 = EZ. | last3 = Serin | first3 = A. | last4 = Yin | first4 = J. | last5 = Cotney | first5 = J. | last6 = Ozduman | first6 = K. | last7 = Avşar | first7 = T. | last8 = Li | first8 = J. | last9 = Murray | first9 = PB. | title = Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. | journal = Science | volume = 339 | issue = 6123 | pages = 1077-80 | month = Mar | year = 2013 | doi = 10.1126/science.1233009 | PMID = 23348505 }}</ref> | |||
*AKT/TRAF7 mutations are usually basal and associated with meningothelial histology. | |||
*KLF4/TRAF7 mutations are highly specific for secretory histology. | |||
*TRAF7 mutations are the first step and occur thorughout the WD40 domain. <ref>{{cite journal |vauthors=Dogan H, Blume C, Patel A, Jungwirth G, Sogerer L, Ratliff M, Ketter R, Herold-Mende C, Jones DTW, Wick W, Vollmuth P, Zweckberger K, Reuss D, von Deimling A, Sahm F |title=Single-cell DNA sequencing reveals order of mutational acquisition in TRAF7/AKT1 and TRAF7/KLF4 mutant meningiomas |journal=Acta Neuropathol |volume=144 |issue=4 |pages=799–802 |date=October 2022 |pmid=35984495 |doi=10.1007/s00401-022-02485-6 |url=}}</ref> | |||
Intraventricular meningiomas have NF2 mutations. | |||
<ref>{{cite journal |vauthors=Jungwirth G, Warta R, Beynon C, Sahm F, von Deimling A, Unterberg A, Herold-Mende C, Jungk C |title=Intraventricular meningiomas frequently harbor NF2 mutations but lack common genetic alterations in TRAF7, AKT1, SMO, KLF4, PIK3CA, and TERT |journal=Acta Neuropathol Commun |volume=7 |issue=1 |pages=140 |date=August 2019 |pmid=31470906 |pmc=6716845 |doi=10.1186/s40478-019-0793-4 |url=}}</ref> | |||
Several inherited diseases are associated with meningiomas: | |||
*[[Neurofibromatosis]] type II<ref>{{Cite journal | last1 = Fontaine | first1 = B. | last2 = Rouleau | first2 = GA. | last3 = Seizinger | first3 = BR. | last4 = Menon | first4 = AG. | last5 = Jewell | first5 = AF. | last6 = Martuza | first6 = RL. | last7 = Gusella | first7 = JF. | title = Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuroma and meningioma). | journal = Ann N Y Acad Sci | volume = 615 | issue = | pages = 338-43 | month = | year = 1991 | doi = | PMID = 2039155 }}</ref> | |||
*Germline SMARCE1 and SMARCB1 mutations<ref>{{Cite journal | last1 = Smith | first1 = MJ. | last2 = O'Sullivan | first2 = J. | last3 = Bhaskar | first3 = SS. | last4 = Hadfield | first4 = KD. | last5 = Poke | first5 = G. | last6 = Caird | first6 = J. | last7 = Sharif | first7 = S. | last8 = Eccles | first8 = D. | last9 = Fitzpatrick | first9 = D. | title = Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. | journal = Nat Genet | volume = 45 | issue = 3 | pages = 295-8 | month = Mar | year = 2013 | doi = 10.1038/ng.2552 | PMID = 23377182 }}</ref><ref>{{Cite journal | last1 = van den Munckhof | first1 = P. | last2 = Christiaans | first2 = I. | last3 = Kenter | first3 = SB. | last4 = Baas | first4 = F. | last5 = Hulsebos | first5 = TJ. | title = Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. | journal = Neurogenetics | volume = 13 | issue = 1 | pages = 1-7 | month = Feb | year = 2012 | doi = 10.1007/s10048-011-0300-y | PMID = 22038540 }}</ref> | |||
*Loss of SUFU (SHH-Pathway).<ref>{{Cite journal | last1 = Aavikko | first1 = M. | last2 = Li | first2 = SP. | last3 = Saarinen | first3 = S. | last4 = Alhopuro | first4 = P. | last5 = Kaasinen | first5 = E. | last6 = Morgunova | first6 = E. | last7 = Li | first7 = Y. | last8 = Vesanen | first8 = K. | last9 = Smith | first9 = MJ. | title = Loss of SUFU function in familial multiple meningioma. | journal = Am J Hum Genet | volume = 91 | issue = 3 | pages = 520-6 | month = Sep | year = 2012 | doi = 10.1016/j.ajhg.2012.07.015 | PMID = 22958902 }}</ref> | |||
*Rare YAP1 fusions in a subset of pediatric meningioma (HIPPO pathyway).<ref>{{Cite journal | last1 = Sievers | first1 = P. | last2 = Chiang | first2 = J. | last3 = Schrimpf | first3 = D. | last4 = Stichel | first4 = D. | last5 = Paramasivam | first5 = N. | last6 = Sill | first6 = M. | last7 = Gayden | first7 = T. | last8 = Casalini | first8 = B. | last9 = Reuss | first9 = DE. | title = YAP1-fusions in pediatric NF2-wildtype meningioma. | journal = Acta Neuropathol | volume = | issue = | pages = | month = Nov | year = 2019 | doi = 10.1007/s00401-019-02095-9 | PMID = 31734728 }}</ref> | |||
Methylation profiling distinguishes two major groups with six distinct clinically relevant methylation classes.<ref>{{Cite journal | last1 = Sahm | first1 = F. | last2 = Schrimpf | first2 = D. | last3 = Stichel | first3 = D. | last4 = Jones | first4 = DT. | last5 = Hielscher | first5 = T. | last6 = Schefzyk | first6 = S. | last7 = Okonechnikov | first7 = K. | last8 = Koelsche | first8 = C. | last9 = Reuss | first9 = DE. | title = DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. | journal = Lancet Oncol | volume = | issue = | pages = | month = Mar | year = 2017 | doi = 10.1016/S1470-2045(17)30155-9 | PMID = 28314689 }}</ref> | |||
===DDx of meningioma & IHC<ref name=pmid16393681>{{cite journal |author=Hahn HP, Bundock EA, Hornick JL |title=Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics |journal=Am. J. Clin. Pathol. |volume=125 |issue=2 |pages=203–8 |year=2006 |month=February |pmid=16393681 |doi=10.1309/G659-FVVB-MG7U-4RPQ |url=http://ajcp.ascpjournals.org/content/125/2/203.full.pdf}}</ref>=== | ===DDx of meningioma & IHC<ref name=pmid16393681>{{cite journal |author=Hahn HP, Bundock EA, Hornick JL |title=Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics |journal=Am. J. Clin. Pathol. |volume=125 |issue=2 |pages=203–8 |year=2006 |month=February |pmid=16393681 |doi=10.1309/G659-FVVB-MG7U-4RPQ |url=http://ajcp.ascpjournals.org/content/125/2/203.full.pdf}}</ref>=== | ||
*S-100 +ve - [[schwannoma]]. | *S-100 strong +ve - [[schwannoma]]. | ||
**+ve in ~80% of fibrous meningiomas. | **+ve in ~80% of fibrous meningiomas. | ||
*CD34 +ve - [[solitary fibrous tumour]]. | *CD34 +ve - [[solitary fibrous tumour]]. | ||
**+ve in ~60% of [[fibrous meningioma]]s. | **+ve in ~60% of [[fibrous meningioma]]s. | ||
*EMA +ve in ~30% of hemangiopericytoma. | *STAT6 nuclear +ve: [[solitary fibrous tumour]]. | ||
*[[EMA]] +ve in ~30% of [[solitary fibrous tumour]]/[[hemangiopericytoma]]. | |||
*Claudin-1 - new kid on the block: +ve in meningioma, but low [[sensitivity]]. | *Claudin-1 - new kid on the block: +ve in meningioma, but low [[sensitivity]]. | ||
*SSTR2A +ve in meningioma, usu. -ve in [[Perineurioma]] <ref>{{Cite journal | last1 = Agaimy | first1 = A. | last2 = Buslei | first2 = R. | last3 = Coras | first3 = R. | last4 = Rubin | first4 = BP. | last5 = Mentzel | first5 = T. | title = Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. | journal = Histopathology | volume = 65 | issue = 1 | pages = 60-70 | month = Jul | year = 2014 | doi = 10.1111/his.12366 | PMID = 24393170 }}</ref> | |||
* Progesterone receptor: +ve in mostly grade I and meningeothelial tumors.<ref>{{Cite journal | last1 = Grunberg | first1 = SM. | title = The role of progesterone receptors in meningioma. | journal = Cancer Treat Res | volume = 58 | issue = | pages = 127-37 | month = | year = 1991 | doi = | PMID = 1683782 }}</ref> | |||
* [[Pulmonary meningothelial-like nodule]] | |||
=== | ===A standard work-up=== | ||
*Ki-67 >5-10% - predicts re-occurrence.<ref>Croul, SE. 8 November 2010.</ref> | *Ki-67 >5-10% - predicts re-occurrence.<ref>Croul, SE. 8 November 2010.</ref> | ||
*PR (progesterone receptor) +ve in > 80% of meningiomas.<ref>{{Cite journal | last1 = Takei | first1 = H. | last2 = Buckleair | first2 = LW. | last3 = Powell | first3 = SZ. | title = Immunohistochemical expression of apoptosis regulating proteins and sex hormone receptors in meningiomas. | journal = Neuropathology | volume = 28 | issue = 1 | pages = 62-8 | month = Feb | year = 2008 | doi = 10.1111/j.1440-1789.2007.00852.x | PMID = 18021195 }}</ref> | *PR (progesterone receptor) +ve in > 80% of meningiomas.<ref>{{Cite journal | last1 = Takei | first1 = H. | last2 = Buckleair | first2 = LW. | last3 = Powell | first3 = SZ. | title = Immunohistochemical expression of apoptosis regulating proteins and sex hormone receptors in meningiomas. | journal = Neuropathology | volume = 28 | issue = 1 | pages = 62-8 | month = Feb | year = 2008 | doi = 10.1111/j.1440-1789.2007.00852.x | PMID = 18021195 }}</ref> | ||
| Line 228: | Line 420: | ||
*[[Neurohistology]]. | *[[Neurohistology]]. | ||
*[[CNS tumours]]. | *[[CNS tumours]]. | ||
*[[Pulmonary meningothelial-like nodule]]. | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category:Neuropathology]] | [[Category:Neuropathology tumours]] | ||
[[Category:Diagnosis]] | [[Category:Diagnosis]] | ||
Latest revision as of 14:13, 19 September 2022
| Meningioma | |
|---|---|
| Diagnosis in short | |
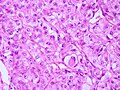
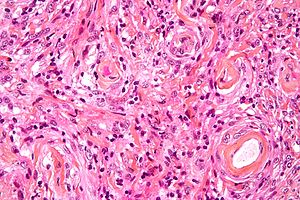 Meningioma. HPS stain. | |
|
| |
| LM | whorled appearance, calcification - psammomatous, +/-nuclear pseudoinclusions |
| Subtypes | Grade I (meningothelial, fibrous, transistional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, metaplastic), Grade II (invasive, clear cell, chordoid), Grade III (papillary, rhabdoid) |
| LM DDx | schwannoma, solitary fibrous tumour, hemangiopericytoma, others |
| IHC | EMA +ve, keratins usu. -ve, CD34 -ve/+ve, S-100 -ve (usu.), PR +ve (-ve in more aggressive ones) |
| Site | see CNS tumours |
|
| |
| Syndromes | neurofibromatosis 2, nevoid basal cell carcinoma syndrome |
|
| |
| Clinical history | +/-radiation |
| Prevalence | common |
| Radiology | extra-axial, intradural lesion, dural tail sign (on MRI) |
| Prognosis | usually benign, dependent on grade |
| Clin. DDx | dependent on site - see CNS tumours |
| Treatment | surgical removal |
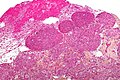
Meningioma a very common tumour in neuropathology.
General
Prevalence
- Most common primary brain tumour.[1]
- May be caused by prior radiation.[2]
- Women develop meningioma twice as likely as men.[3]
- More than 90% are solitary.
Prognosis
- Most are benign - usu. a good prognosis.
- Even benign tumors may show extensive local spread - considerable morbidity and mortality.
- Metastases are rare and then usu. after surgery.
- May be malignant - bad prognosis.
- Factors associated with unfavourable prognosis:
Genetics
- May be seen in genetic disorders such as:
- Neurofibromatosis 2 (NF2).[7]
- Nevoid basal cell carcinoma syndrome (Gorlin syndrome).[8][9]
Quick overview
| Name | Histologic criteria | Subtypes | Image |
|---|---|---|---|
| Classic, WHO I | less then 4 mit/10 HPF and no atypia | meningeothelial, fibroblastic, transitional, psammomatous, angiomatous, microcytsic, secretory, lymphoplasmacyte-rich, metaplastic | |
| Atypical, WHO II | brain invasion, 4 or more mit/10 HPF, or 3 of the following: necrosis, increased cellularity, high nuc:cyto ratio, nucleoli, sheeting | chordoid, clear cell | |
| Anaplastic, WHO III | 20 or more mitoses/10 HPF, morphologiy similiar to carcinoma or sarcoma | rhabdoid, papillary |
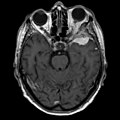
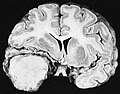
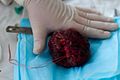
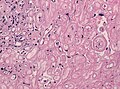
Gross/Radiology
- Extra-axial, intradural.
- Can be extradural - very rare.[10]
- Dural tail sign (DTS) on MRI.[11][12]
- +/-Hyperostosis.
- Associated with invasion into the skull in ~20% of cases.[15]
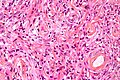
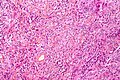
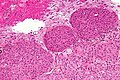
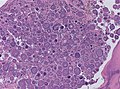
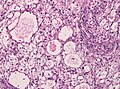
Microscopic
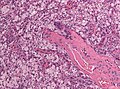
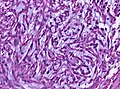
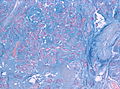
Features (memory device WCN):
- Whorled appearance - key feature.
- Calcification, psammomatous (target-like appearance; (tight) onion skin).
- +/-Nuclear pseudoinclusions - focal nuclear clearing with a sharp interface to unremarkable chromatin.
Notes:
- May involute into benign sclerotic tissue.[16]
- Thick-walled blood vessels -> think schwannoma.
DDx:
- Schwannoma - especially at CP angle.
- Solitary fibrous tumour.
- Hemangiopericytoma.
- Others - see subtypes.
Images
www:
Morphologic subtypes
- Many subtypes exist.[17]
- The histologic subtypes generally don't have much prognostic significance.
- Some subtypes are high grade by definition; also see histologic grading.
Grade I
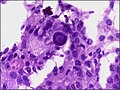
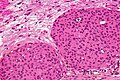
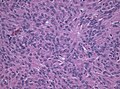
Meningothelial meningioma
- Most common.
Microscopic:
- Syncytial, nuclear clearing (pseudoinclusions).
- Whorls, Onion bulb formations.
- Few psammoma bodies.
Molecular:
- AKT E17K mutations.[18]
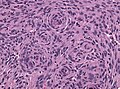
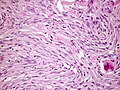
Fibrous meningioma
- AKA fibroblastic meningioma.
- Not collagen... but looks like it.
- It is really laminin or fibronectin.
- Spindle cells in parallel bundles.
- Few to none whorl formations.
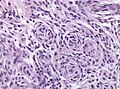
Transitional meningioma
- AKA mixed.
- Common.
- Lobular and fasicular growth patterns coexist.
- Usu. a mixture of meningeothelial and fibromatous meningioma
Psammomatous meningioma
Microscopic:
- Psammoma bodies dominate over tumor cells.
- Irregular calcifications (confluent psammoma bodies).
- Usually found in spinal cord.
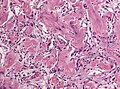
Angiomatous meningioma
- AKA vascular.
- May bleed like stink.
- May show extensive edema.
- Hyalinized vessels dominate over tumor cells.
- Degenerative nuclear atypia.
DDx:
- Vascular malformatons
- Hemangioblastoma
Microcystic meningioma
Microscopic:
- Cystic appearance.
- Increased cytologic pleomorphism of the elongated cells.
DDx:
- Clear cell meningioma
- Hemangioblastoma
Secretory meningioma
- Associated with brain edema; may have a worse outcome.
Microscopic:[19]
Molecular:
- Combined KLF4 K409Q and TRAF7 mutations.[20]
DDx:
- Metastatic mucinous adenocarcinoma.
- Pituitary adenoma
Images:
Lymphoplasmacyte-rich meningioma
Microscopic:
- Lymphocytes.
- Plasma cells.
Images:
- Lymphoplasmacyte-rich meningioma - case 1 - several images (upmc.edu).
- Lymphoplasmacyte-rich meningioma - case 2 - several images (upmc.edu).
- Lymphoplasmacyte-rich meningioma - case 3 - several images (upmc.edu).
Metaplastic meningioma
- No clinical significance.
- Probably do not represent true metaplasia in all cases.
- Clincal information is rquired to distinguish between bone invasion and meningiomas with bone formation.
Microscopic:
- Cartilage or bone formation.
- Myxoid or xanthomatous changes.
Grade II
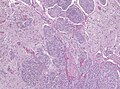
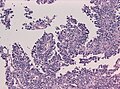
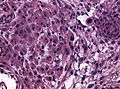
Brain invasive meningioma
- Invades the brain (irregular, tongue-like).
- Absence of leptomeningeal layer.
- Brain invasion can be present in grade I tumors, these are then classified as "atypical", ie. as grade II tumors.
- The prognostic significance of brain invasion is still unclear, some studies do not show a course similiar to grade II meningiomas.[22][23][24]
Images:
Clear cell meningioma
Epidemiology:
- Usu. spinal cord.[25]
Microscopic:
- Clear cells - contain glycogen (PAS +ve).
Molecular:
- SMARCE1 mutations.[26]
Images:
Chordoid meningioma
- Chordoma-like.
Microscopic:
- Myxoid appearance.
Image:
Grade III
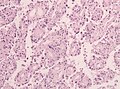
Papillary meningioma
Microscopic:
- discohesive meningothelial tumour cells around a fibrovascular core.
- perivascular pseudorosettes.
Rhabdoid meningioma
Microscopic:
- Rhabdoid appearance (abundant cytoplasm).
- Cross-striations.
www:
- Rhabdoid meningioma - case 1 - several images (upmc.edu).
- Rhabdoid meningioma - case 2 - several images (upmc.edu).
Other morphological variants
These are currently not listed in the WHO as separate entities.
Histologic grading
Grading:[17]
- Grade 1:
- Low mitotic rate (< 4 mitoses/10 HPF - for whatever HPF means, see HPFitis).
- Excludes clear cell, chordoid, papillary, and rhabdoid subtypes.
- Grade 2 (either #1, #2 or #3):
- Brain-invasive meningioma.
- Invasion of meningioma into brain.
- Meninogioma with entraped GFAP +ve tissue.
- Invasion of meningioma into brain.
- Atypical meningioma (by histomorphology) - either A or B.
- A. Intermediate mitotic rate (>= 4 mitoses/10 HPF - for whatever HPF means, see HPFitis.)
- B. Three of the following five features:
- Clear cell or chordoid subtype.
- Brain-invasive meningioma.
- Grade 3 (either of the following):
- High mitotic rate (>=20 mitoses/10 HPF - for whatever HPF means, see HPFitis.)
- "Frank anaplasia"; marked nuclear atypia.
- Papillary or rhabdoid subtype.
Notes:
- Grade II soft criteria memory device HMNs: hypercellular, macronucleoli, NC ratio increased, necrosis, sheeting.
IHC
- EMA +ve (approx. 90%).[30]
- PR +ve (approx. 75%, expression decreases from grade I to III).
- SSTR2A +ve (approx. 95%).
- S100 variable (up to 35% cases, usually patchy).[31]
- SOX10 -ve.
- GFAP -ve.
- CD34 usu. -ve (approx 8% cases positive).
- CD13 +ve.[32]
- Other CKs usually -ve (approx 6% cases positive, mostly secretory meningiomas).
Molecular
Non-syndromal meningiomas may show AKT1/TRAF7, SMO, KLF4/TRAF7, and PIK3CA mutations (1/3 of cases).[33]
- AKT/TRAF7 mutations are usually basal and associated with meningothelial histology.
- KLF4/TRAF7 mutations are highly specific for secretory histology.
- TRAF7 mutations are the first step and occur thorughout the WD40 domain. [34]
Intraventricular meningiomas have NF2 mutations. [35]
Several inherited diseases are associated with meningiomas:
- Neurofibromatosis type II[36]
- Germline SMARCE1 and SMARCB1 mutations[37][38]
- Loss of SUFU (SHH-Pathway).[39]
- Rare YAP1 fusions in a subset of pediatric meningioma (HIPPO pathyway).[40]
Methylation profiling distinguishes two major groups with six distinct clinically relevant methylation classes.[41]
DDx of meningioma & IHC[42]
- S-100 strong +ve - schwannoma.
- +ve in ~80% of fibrous meningiomas.
- CD34 +ve - solitary fibrous tumour.
- +ve in ~60% of fibrous meningiomas.
- STAT6 nuclear +ve: solitary fibrous tumour.
- EMA +ve in ~30% of solitary fibrous tumour/hemangiopericytoma.
- Claudin-1 - new kid on the block: +ve in meningioma, but low sensitivity.
- SSTR2A +ve in meningioma, usu. -ve in Perineurioma [43]
- Progesterone receptor: +ve in mostly grade I and meningeothelial tumors.[44]
- Pulmonary meningothelial-like nodule
A standard work-up
- Ki-67 >5-10% - predicts re-occurrence.[45]
- PR (progesterone receptor) +ve in > 80% of meningiomas.[46]
- Loss of PR staining predicts recurrence.
- Strong association with tumour grade:[47]
- Low WHO grade tumours usu. +ve.
- High WHO grade tumours usu. -ve.
See also
References
- ↑ Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, TJ.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, DC. et al. (Oct 2014). "Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review.". J Neurosurg: 1-20. doi:10.3171/2014.7.JNS131644. PMID 25343186.
- ↑ Baldi, I.; Engelhardt, J.; Bonnet, C.; Bauchet, L.; Berteaud, E.; Grüber, A.; Loiseau, H. (Sep 2014). "Epidemiology of meningiomas.". Neurochirurgie. doi:10.1016/j.neuchi.2014.05.006. PMID 25249493.
- ↑ Wiemels, J.; Wrensch, M.; Claus, EB. (Sep 2010). "Epidemiology and etiology of meningioma.". J Neurooncol 99 (3): 307-14. doi:10.1007/s11060-010-0386-3. PMID 20821343.
- ↑ Shankar, GM.; Abedalthagafi, M.; Vaubel, RA.; Merrill, PH.; Nayyar, N.; Gill, CM.; Brewster, R.; Bi, WL. et al. (04 2017). "Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas.". Neuro Oncol 19 (4): 535-545. doi:10.1093/neuonc/now235. PMID 28170043.
- ↑ Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D. et al. (May 2016). "TERT Promoter Mutations and Risk of Recurrence in Meningioma.". J Natl Cancer Inst 108 (5). doi:10.1093/jnci/djv377. PMID 26668184.
- ↑ Katz, LM.; Hielscher, T.; Liechty, B.; Silverman, J.; Zagzag, D.; Sen, R.; Wu, P.; Golfinos, JG. et al. (Apr 2018). "Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence.". Acta Neuropathol. doi:10.1007/s00401-018-1844-9. PMID 29627952.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/neurotest/Q13-Ans.htm. Accessed on: 26 October 2010.
- ↑ Kimonis, VE.; Mehta, SG.; Digiovanna, JJ.; Bale, SJ.; Pastakia, B.. "Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome.". Genet Med 6 (6): 495-502. doi:10.109701.GIM.0000145045.17711.1C. PMID 15545745.
- ↑ Lee, CW.; Tan, TC. (Feb 2014). "Meningioma associated with Gorlin's syndrome.". J Clin Neurosci 21 (2): 349-50. doi:10.1016/j.jocn.2013.02.033. PMID 24100109.
- ↑ URL: http://path.upmc.edu/cases/case702.html. Accessed on: 2 February 2012.
- ↑ Ikeda, D.; Chiocca, EA. (Oct 2012). "Editorial: dural tail sign.". J Neurosurg 117 (4): 643-4. doi:10.3171/2012.2.JNS12266. PMID 22839655.
- ↑ Wen, M.; Jung, S.; Moon, KS.; Pei, J.; Lee, KH.; Jin, SG.; Li, SY.; Ryu, HH. (Dec 2014). "Immunohistochemical profile of the dural tail in intracranial meningiomas.". Acta Neurochir (Wien) 156 (12): 2263-73. doi:10.1007/s00701-014-2216-4. PMID 25238986.
- ↑ Aoki, S.; Sasaki, Y.; Machida, T.; Tanioka, H.. "Contrast-enhanced MR images in patients with meningioma: importance of enhancement of the dura adjacent to the tumor.". AJNR Am J Neuroradiol 11 (5): 935-8. PMID 2120998.
- ↑ Qi, ST.; Liu, Y.; Pan, J.; Chotai, S.; Fang, LX. (Oct 2012). "A radiopathological classification of dural tail sign of meningiomas.". J Neurosurg 117 (4): 645-53. doi:10.3171/2012.6.JNS111987. PMID 22839654.
- ↑ Goyal, N.; Kakkar, A.; Sarkar, C.; Agrawal, D.. "Does bony hyperostosis in intracranial meningioma signify tumor invasion? A radio-pathologic study.". Neurol India 60 (1): 50-4. doi:10.4103/0028-3886.93589. PMID 22406780.
- ↑ URL: http://radiographics.rsna.org/content/23/3/785.long. Accessed on: 3 November 2010.
- ↑ 17.0 17.1 Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 194. ISBN 978-0443069826.
- ↑ Sahm, F.; Bissel, J.; Koelsche, C.; Schweizer, L.; Capper, D.; Reuss, D.; Böhmer, K.; Lass, U. et al. (Nov 2013). "AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry.". Acta Neuropathol 126 (5): 757-62. doi:10.1007/s00401-013-1187-5. PMID 24096618.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm. Accessed on: 12 October 2011.
- ↑ Reuss, DE.; Piro, RM.; Jones, DT.; Simon, M.; Ketter, R.; Kool, M.; Becker, A.; Sahm, F. et al. (Mar 2013). "Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations.". Acta Neuropathol 125 (3): 351-8. doi:10.1007/s00401-013-1093-x. PMID 23404370.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm. Accessed on: 3 January 2012.
- ↑ Baumgarten, P.; Gessler, F.; Schittenhelm, J.; Skardelly, M.; Tews, DS.; Senft, C.; Dunst, M.; Imoehl, L. et al. (Sep 2016). "Brain invasion in otherwise benign meningiomas does not predict tumor recurrence.". Acta Neuropathol 132 (3): 479-81. doi:10.1007/s00401-016-1598-1. PMID 27464983.
- ↑ Brokinkel, B.; Hess, K.; Mawrin, C. (Apr 2017). "Brain invasion in Meningiomas - Clinical considerations and impact of neuropathological evaluation: A systematic Review.". Neuro Oncol. doi:10.1093/neuonc/nox071. PMID 28419308.
- ↑ Pizem, J.; Velnar, T.; Prestor, B.; Mlakar, J.; Popovic, M.. "Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen.". Clin Neuropathol 33 (5): 354-63. doi:10.5414/NP300750. PMID 25034703.
- ↑ Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 200. ISBN 978-0443069826.
- ↑ Smith, MJ.; Wallace, AJ.; Bennett, C.; Hasselblatt, M.; Elert-Dobkowska, E.; Evans, LT.; Hickey, WF.; van Hoff, J. et al. (Dec 2014). "Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas.". J Pathol 234 (4): 436-40. doi:10.1002/path.4427. PMID 25143307.
- ↑ Zunarelli, E.; Tallarico, E.; Valentini, A.; Maiorana, A. (2010). "Oncocytic meningioma: study of eight new cases and analysis of 13 reported cases.". Pathology 42 (6): 587-9. doi:10.3109/00313025.2010.508740. PMID 20854082.
- ↑ Haberler, C.; Jarius, C.; Lang, S.; Rössler, K.; Gruber, A.; Hainfellner, JA.; Budka, H. (Feb 2002). "Fibrous meningeal tumours with extensive non-calcifying collagenous whorls and glial fibrillary acidic protein expression: the whorling-sclerosing variant of meningioma.". Neuropathol Appl Neurobiol 28 (1): 42-7. PMID 11849562.
- ↑ Liverman, C.; Mafra, M.; Chuang, SS.; Shivane, A.; Chakrabarty, A.; Highley, R.; Hilton, DA.; Byrne, NP. et al. (Aug 2015). "A clinicopathologic study of 11 rosette-forming meningiomas: a rare and potentially confusing pattern.". Acta Neuropathol 130 (2): 311-3. doi:10.1007/s00401-015-1456-6. PMID 26106026.
- ↑ Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 13. ISBN 978-0443069826.
- ↑ "The prognostic role of the immunohistochemical expression of S100 in meningiomas". J Cancer Res Clin Oncol. July 2022. doi:10.1007/s00432-022-04186-9. PMID 35838837.
- ↑ "CD13 is a useful tool in the differential diagnosis of meningiomas with potential biological and prognostic implications". Virchows Arch 480 (6): 1223–1230. June 2022. doi:10.1007/s00428-022-03304-9. PMC 9184408. PMID 35212813. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9184408/.
- ↑ Clark, VE.; Erson-Omay, EZ.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avşar, T.; Li, J. et al. (Mar 2013). "Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO.". Science 339 (6123): 1077-80. doi:10.1126/science.1233009. PMID 23348505.
- ↑ "Single-cell DNA sequencing reveals order of mutational acquisition in TRAF7/AKT1 and TRAF7/KLF4 mutant meningiomas". Acta Neuropathol 144 (4): 799–802. October 2022. doi:10.1007/s00401-022-02485-6. PMID 35984495.
- ↑ "Intraventricular meningiomas frequently harbor NF2 mutations but lack common genetic alterations in TRAF7, AKT1, SMO, KLF4, PIK3CA, and TERT". Acta Neuropathol Commun 7 (1): 140. August 2019. doi:10.1186/s40478-019-0793-4. PMC 6716845. PMID 31470906. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6716845/.
- ↑ Fontaine, B.; Rouleau, GA.; Seizinger, BR.; Menon, AG.; Jewell, AF.; Martuza, RL.; Gusella, JF. (1991). "Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuroma and meningioma).". Ann N Y Acad Sci 615: 338-43. PMID 2039155.
- ↑ Smith, MJ.; O'Sullivan, J.; Bhaskar, SS.; Hadfield, KD.; Poke, G.; Caird, J.; Sharif, S.; Eccles, D. et al. (Mar 2013). "Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas.". Nat Genet 45 (3): 295-8. doi:10.1038/ng.2552. PMID 23377182.
- ↑ van den Munckhof, P.; Christiaans, I.; Kenter, SB.; Baas, F.; Hulsebos, TJ. (Feb 2012). "Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri.". Neurogenetics 13 (1): 1-7. doi:10.1007/s10048-011-0300-y. PMID 22038540.
- ↑ Aavikko, M.; Li, SP.; Saarinen, S.; Alhopuro, P.; Kaasinen, E.; Morgunova, E.; Li, Y.; Vesanen, K. et al. (Sep 2012). "Loss of SUFU function in familial multiple meningioma.". Am J Hum Genet 91 (3): 520-6. doi:10.1016/j.ajhg.2012.07.015. PMID 22958902.
- ↑ Sievers, P.; Chiang, J.; Schrimpf, D.; Stichel, D.; Paramasivam, N.; Sill, M.; Gayden, T.; Casalini, B. et al. (Nov 2019). "YAP1-fusions in pediatric NF2-wildtype meningioma.". Acta Neuropathol. doi:10.1007/s00401-019-02095-9. PMID 31734728.
- ↑ Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, DT.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C. et al. (Mar 2017). "DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis.". Lancet Oncol. doi:10.1016/S1470-2045(17)30155-9. PMID 28314689.
- ↑ Hahn HP, Bundock EA, Hornick JL (February 2006). "Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics". Am. J. Clin. Pathol. 125 (2): 203–8. doi:10.1309/G659-FVVB-MG7U-4RPQ. PMID 16393681. http://ajcp.ascpjournals.org/content/125/2/203.full.pdf.
- ↑ Agaimy, A.; Buslei, R.; Coras, R.; Rubin, BP.; Mentzel, T. (Jul 2014). "Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel.". Histopathology 65 (1): 60-70. doi:10.1111/his.12366. PMID 24393170.
- ↑ Grunberg, SM. (1991). "The role of progesterone receptors in meningioma.". Cancer Treat Res 58: 127-37. PMID 1683782.
- ↑ Croul, SE. 8 November 2010.
- ↑ Takei, H.; Buckleair, LW.; Powell, SZ. (Feb 2008). "Immunohistochemical expression of apoptosis regulating proteins and sex hormone receptors in meningiomas.". Neuropathology 28 (1): 62-8. doi:10.1111/j.1440-1789.2007.00852.x. PMID 18021195.
- ↑ Tao, Y.; Liang, G.; Li, Z.; Wang, Y.; Wu, A.; Wang, H.; Lu, Y.; Liu, Z. et al. (May 2012). "Clinical features and immunohistochemical expression levels of androgen, estrogen, progesterone and Ki-67 receptors in relationship with gross-total resected meningiomas relapse.". Br J Neurosurg. doi:10.3109/02688697.2012.685780. PMID 22616825.